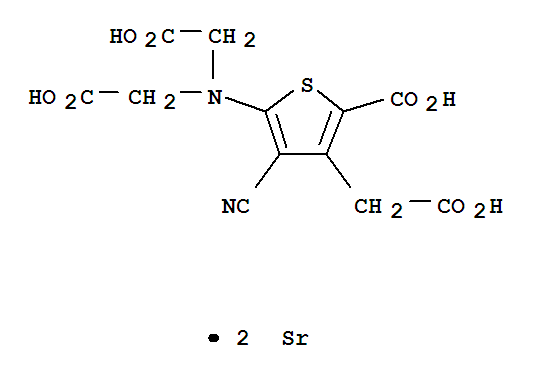
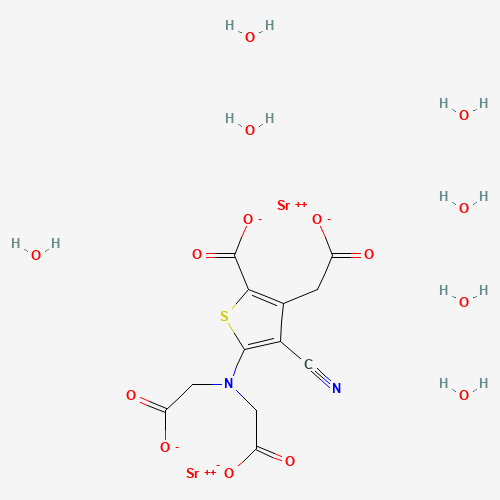
Strontium ranelate Good Manufacturer supply High Quality 135459-87-9
- Molecular Formula:C12H10N2O8SSr2
- Molecular Weight:517.52
- Appearance/Colour:crystalline solid
- Melting Point:>310°C (dec.)
- Boiling Point:778.8 °C at 760 mmHg
- Flash Point:424.8 °C
- PSA:160.47000
- LogP:-0.32272
Strontium ranelate (135459-87-9) Usage
Strontium ranelate, a divalent strontium salt of ranelic acid, has been developed and launched for the treatment of osteoporosis. As early as 1910, investigations suggested that strontium stimulates the formation of osteoid tissues while simultaneously repressing the resorptive process in bones. Specifically, strontium enhances pre-osteoblastic cell replication, inhibits pre-osteoclast differentiation, and suppresses the bone-resorbng activity of osteoclasts. From the evaluation of 26 strontium salts, ranelic acid was selected as the ideal strontium carrier due to its physicochemical and pharmacokinetic properties. The thiophene core of ranelic acid is constructed by the condensation of dialkyl 3-oxoglutarate, malononitrile, and sulfur in a suitable alcohol in the presence of morpholine or diethylamine. The resultant diester of 5-amino-3-carboxymethyl-4-cyano-2-thiophenecarboxylic acid is subsequently dialkylated with an alkyl bromoacetate to provide the tetraester precursor to strontium ranelate. Strontium ranelate is supplied in a 2 g sachet, and the drug is evenly suspended in water prior to consumption. Since the simultaneous ingestion of either calcium or food has a negative influence on the bioavailability of strontium ranelate, it is recommended that strontium ranelate be administered once a day at bedtime. Following this regimen, the absolute bioavailability of strontium is 27% while that of ranelic acid is 2.5%. Because strontium ranelate dissociates after intake, and ranelic acid has negligible absorption, the effects of the drug on bone metabolism are dependent on the pharmacokinetics of strontium. In postmenopausal women, the half-life of strontium is 6.3±2.3 days, and renal clearance accounts for 57%of the total clearance of 12mL/min. After a 25-day treatment, the maximum plasma concentration of strontium is 20±2.3 mg/L. In addition, not only is perfect stability of strontium plasma concentration achieved within 3 to 24 months of chronic administration so is stabilization of strontium incorporation into bones. Strontium is incorporated into bone by two mechanisms. The predominant mode involves the rapid, saturable surface exchange with calcium. A slower mechanism embodies the incorporation of strontium into the crystal lattice of the bone mineral; however, only a small amount of calcium in the apatite is substituted by strontium at pharmacological doses. A phase II clinical trial assessed the effect of various strontium ranelate doses in postmenopausal women with established osteoporosis. The primary efficacy endpoint for this double-blind, randomized, placebo- controlled trial was the measure of mean lumbar bone mineral density (BMD) by dual-energy X-ray absorptiometry. A statistically significant dose-dependent increase in lumbar BMD was observed; increases of 1.3, 5.9, 8.3, and 13.6% were recorded for placebo, 500-, 1000-, and 2000-mg doses of strontium ranelate, respectively. In a phase III trial encompassing 1,649 osteoporotic postmenopausal women from 12 countries, the efficacy of a 2 g/day dose in preventing new vertebral fractures was evaluated. The mean lumbar BMD was 0.73 g/cm2 while the mean age at baseline was 70 years. All of the enrolled patients had at least one prior vertebral fracture. The primary end point for this study was a reduction in the incidence of patients experiencing fractures. While 222 women in the placebo group experienced a new vertebral fracture, only 139 patients treated with strontium ranelate presented with new fractures. Furthermore, the risk of fracture was reduced by 51% in the third year alone, implicating the sustained efficacy of the drug. For both the phase II and phase III studies, strontium ranelate was well tolerated with most of the adverse events being mild-to-moderate in severity. The most commonly reported events in all treatment groups were musculoskeletal disorders (back pain, arthralgia, and lumbar pain). As for laboratory measurements, only creatine phosphokinase, the musculoskeletal isoenzyme, was significantly elevated in the 1000-mg and 2000-mg strontium ranelate groups; however, this did not translate into any particular clinical or biological abnormality. Without relevant data regarding bone safety in patients with renal impairment, strontium ranelate is currently contraindicated in patients with creatine clearance below 30mL/min.
How to get the best price on Strontium ranelate?
Zibo Hangyu Biotechnology Development Co., Ltd is a quality supplier and manufacturer of Strontium ranelate . You can buy high quality, low price Strontium ranelate 135459-87-9 here.
Zibo Hangyu Biotechnology Development Co., Ltd. is a comprehensive chemical enterprise focusing on fine chemical products and pharmaceutical intermediates, integrating research and development, production and sales. The company is equipped with advanced equipment quality inspection and research center. At the same time, the company has excellent talents with rich experience in process development and quality control. To provide customers with total solutions from raw material processing to chemical synthesis systems. The company has established a standardized and perfect quality standard system to provide stable and high quality production and service.

Milestones
- 2012yearThe company was founded, began to engage in the production and supply of chemical raw materials
- 2014yearExpand the scale of production, pharmaceutical raw materials production workshop was established
- 2016yearCooperated with domestic and foreign chemical platforms to promote self-produced and high-end pharmaceutical intermediates
- 2019yearIn recent years to participate in the international pharmaceutical raw materials exhibitions
- 2023yearBusiness continues to expand, Production and Sales teams continue to grow
- 2026yearPlans to set up foreign branches