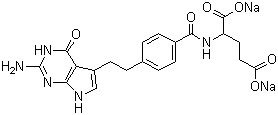
Pemetrexed disodium Good Manufacturer supply High Quality 150399-23-8
- Molecular Formula:C20H19N5Na2O6
- Molecular Weight:471.37
- Appearance/Colour:crystalline solid
- Melting Point:36-38°C
- Boiling Point:160°C 20mm
- Flash Point:160°C/20mm
- PSA:196.92000
- LogP:-1.03090
Pemetrexed disodium (150399-23-8) Usage
Pemetrexed, a pyrrolo[2,3-d]pyrimidine-based antifolate that disrupts cell replication by inhibiting multiple folate-dependent metabolic processes, was initially developed and launched in the US for the treatment of malignant pleural mesothelioma in conjunction with cisplatin. Patients who are not candidates for surgery may benefit from this combination therapy. Clinical data demonstrated that the median overall survival time increased to 12.1 months, compared with 9.3 months for patients receiving cisplatin alone. In August of 2004, the FDA also approved pemetrexed as a second-line treatment of non-small-cell lung cancer (NSCLC). While median survival is comparable to the standard second-line treatment docetaxel, the improved toxicity profile (significant reduction in neutropenia) accelerated the approval for NSCLC. Its effectiveness as an anticancer drug is derived from its ability to gain internal cell access via the reduced folate carrier and membrane folate binding protein transport systems. Once inside, pemetrexed undergoes polyglutamation, and the resultant polyglutamate forms (predominantly the pentaglutamate) inhibit the folate-dependent enzymes thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT). Against recombinant human TS, pemetrexed has a Ki of 109nM while the triglutamate and pentaglutamate forms have Ki values of 1.6nM and 1.3 nM, respectively. All forms of pemetrexed display similar potency against recombinant human DHFR (7 nM), but the pentaglutamate form is significantly more potent against recombinant murine GARFT than the parent (Ki=65nM versus 9.3μM). The selectivity of pemetrexed may be explained by the fact that polyglutamation is more likely to occur in cancer cells compared to normal cells while its prolonged duration of action may be attributed to decreased cellular efflux of the polyglutamate forms. While several different routes have provided pemetrexed, one of the most efficient exploits the propensity of 2,6-diamino-3Hpyrimidin- 4-one to undergo Michael additions at its unsubstituted C-5 position. Using ethyl 4-(4-nitrobut-3-enyl)benzoate as the Michael acceptor, the resulting adduct is then converted to the ultimate precursor for glutamyl coupling via a onepot, three-step process (Nef reaction to transform the nitro to the aldehyde, intramolecular condensation to afford the pyrrole, and saponification of the ethyl ester). A typical treatment regimen involves intravenous administration of pemetrexed, infused over ten minutes, at a dose of 500mg/m2 followed by a thirty minute wash-out period and then cisplatin intravenously over two hours at a dose of 75mg/m2. Both drugs are given on Day 1 of a 21-day cycle. In order to reduce treatment-related hematological and GI toxicity, patients are instructed to take folic acid and vitamin B12 as a prophylactic measure. Pretreatment with a corticosteroid is also recommended to prevent possible skin rashes. Pemetrexed is primarily excreted intact in the urine, with 70–90% of the dose being recovered within 24 hours of administration. The half-life of pemetrexed is 3.5 hours in patients with normal renal function, and the total systemic clearance is 91.8mL/min. As expected, clearance decreases as renal impairment increases. The drug’s plasma protein binding is 81%, and it has a steady state volume of distribution of 16.1 L. The pharmacokinetics of pemetrexed is linear with dose and remains unchanged over multiple treatment cycles. While in vitro studies suggest that pemetrexed would not interfere with drugs metabolized by CYP3A4, CYP2D6, CYP2C9, and CYP1A2, ibuprofen (400mg q.d.) does reduce pemetrexed clearance by 20%. Caution should, therefore, be taken when administering pemetrexed concurrently with ibuprofen to patients with renal insufficiency and should not be given at all to patients whose creatinine clearance is <45mL/min. .
How to get the best price on Pemetrexed disodium?
Zibo Hangyu Biotechnology Development Co., Ltd is a quality supplier and manufacturer of Pemetrexed disodium . You can buy high quality, low price Pemetrexed disodium 150399-23-8 here.
Zibo Hangyu Biotechnology Development Co., Ltd. is a comprehensive chemical enterprise focusing on fine chemical products and pharmaceutical intermediates, integrating research and development, production and sales. The company is equipped with advanced equipment quality inspection and research center. At the same time, the company has excellent talents with rich experience in process development and quality control. To provide customers with total solutions from raw material processing to chemical synthesis systems. The company has established a standardized and perfect quality standard system to provide stable and high quality production and service.

Milestones
- 2012yearThe company was founded, began to engage in the production and supply of chemical raw materials
- 2014yearExpand the scale of production, pharmaceutical raw materials production workshop was established
- 2016yearCooperated with domestic and foreign chemical platforms to promote self-produced and high-end pharmaceutical intermediates
- 2019yearIn recent years to participate in the international pharmaceutical raw materials exhibitions
- 2023yearBusiness continues to expand, Production and Sales teams continue to grow
- 2026yearPlans to set up foreign branches