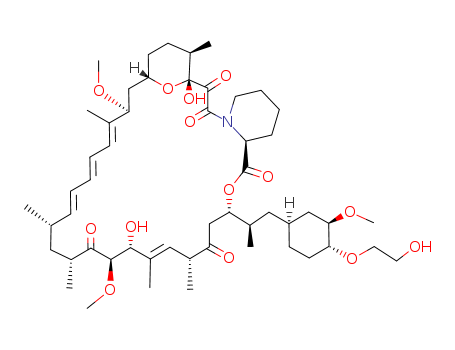
Everolimus Good Manufacturer supply High Quality 159351-69-6
- Molecular Formula:C53H83NO14
- Molecular Weight:958.24
- Appearance/Colour:off white solid
- Vapor Pressure:0mmHg at 25°C
- Melting Point:NA
- Refractive Index:1.548
- Boiling Point:998.7 °C at 760 mmHg
- PKA:10.40±0.70(Predicted)
- Flash Point:557.8 °C
- PSA:204.66000
- Density:1.18 g/cm3
- LogP:6.13510
Everolimus (159351-69-6) Usage
Everolimus, an oral immunosuppressant for the treatment of kidney and heart transplant rejection, is the 40-O-(2-hydroxyethyl) derivative of rapamycin. It has immunosuppressive properties similar to those of rapamycin, but with improved pharmacokinetic profile. In addition, the 40-O-(2-hydroxyethyl) group alters the physico-chemical properties of the macrolide to allow galenic formulation. Everolimus is prepared in a two-step semisynthesis starting from rapamycin, by alkylation of the 40-hydroxyl group with t-butyldimethylsilyloxyethyl triflate and subsequent cleavage of the silyl protecting group. Everolimus, like rapamycin, is a proliferation signal inhibitor that exerts its immunosuppressive effect by inhibiting the activation of p70 S6 kinase, thereby blocking growth factor-driven proliferation of T cells, B cells and vascular smooth muscle cells, and arresting cell cycle at the G1 phase. Inhibition of p70 S6 kinase activation by everolimus and rapamycin is mediated by their binding to FKBP12 (FK506 binding-protein 12). Everolimus inhibits FK506 binding to FKBP12 with an IC50 of 1.8–2.6 nM, and it is about 3- to 5-fold less potent than rapamycin (IC50=0.4–0.9 nM). The in vitro immunosuppressive activity of everolimus is also slightly less than that of rapamycin as demonstrated in a mixed lymphocyte reaction (MLR) assay (IC50=0.2–1.6 nM versus 0.07–0.5 nM, respectively) and in antigen-specific human helper T-cell clones (IC50=0.05–0.17nM versus 0.014–0.37nM, respectively). However, the in vivo immunosuppressive activity of oral everolimus 1–5 mg/ kg/day is similar to that of rapamycin at equivalent doses in rat models of renal or cardiac transplantation, localized graft-versus-host disease, and autoimmune glomerulonephritis. The recommended dosage of everolimus is 0.75 mg twice daily, and it is used in combination with cyclosporine microemulsion and corticosteroids. Following oral dosing, the peak concentration (Cmax) of everolimus is estimated between 1.5 to 2 hours, and steady state is achieved within 4 days. The terminal elimination half-life is 21 to 35 hours. By comparison, rapamycin has a longer elimination half-life (60 hours) and longer time to reach steady state (7 to 14 days). Consequently, rapamycin treatment requires a large loading dose, followed by once daily maintenance dose, whereas everolimus is administered twice daily but without the need of a loading dose. Everolimus is extensively metabolized, primarily by CYP3A4. Approximately 80% of the dose is excreted in the feces and about 5% in the urine. In clinical trials with adult cardiac transplant recipients, oral everolimus 0.75 or 1.5 mg twice daily significantly reduced the incidence of efficacy failure as well as cardiac allograft vasculopathy (CAV) up to 2 years after transplantation as compared with azathioprene 1–3 mg/kg/day. However, graft and patient survival rates at 1 year were similar in patients receiving everolimus and azathioprene. In trials involving renal transplant recipients, the combined incidence of biopsy-confirmed acute rejection, graft loss, death, or loss to follow-up was similar in patients receiving everolimus 1.5 or 3 mg/day or mycophenolate mofetil 2 g/day up to 3 years after transplantation. Everolimus was well tolerated in transplant patients. The incidence of viral infection including cytomegalovirus (CMV) was reduced in comparison to azathioprene and mycophenolate mofetil, but bacterial infections were more frequent. Main adverse events associated with everolimus were thrombocytopenia, leucopenia, and elevated serum lipids and creatinine.
How to get the best price on Everolimus?
Zibo Hangyu Biotechnology Development Co., Ltd is a quality supplier and manufacturer of Everolimus . You can buy high quality, low price Everolimus 159351-69-6 here.
Zibo Hangyu Biotechnology Development Co., Ltd. is a comprehensive chemical enterprise focusing on fine chemical products and pharmaceutical intermediates, integrating research and development, production and sales. The company is equipped with advanced equipment quality inspection and research center. At the same time, the company has excellent talents with rich experience in process development and quality control. To provide customers with total solutions from raw material processing to chemical synthesis systems. The company has established a standardized and perfect quality standard system to provide stable and high quality production and service.

Milestones
- 2012yearThe company was founded, began to engage in the production and supply of chemical raw materials
- 2014yearExpand the scale of production, pharmaceutical raw materials production workshop was established
- 2016yearCooperated with domestic and foreign chemical platforms to promote self-produced and high-end pharmaceutical intermediates
- 2019yearIn recent years to participate in the international pharmaceutical raw materials exhibitions
- 2023yearBusiness continues to expand, Production and Sales teams continue to grow
- 2026yearPlans to set up foreign branches




![4,7-DIAZA-SPIRO[2.5]OCTANE-4-CARBOXYLIC ACID TERT-BUTYL ESTER](/upload/2024/6/c5ec77ca-ad0d-49aa-ac7b-af695c23101c.png)